Compounds of zinc and cadmium with photoluminescent properties
During the last years, the synthesis of coordination compounds (metal plus organic molecules) has aroused great interest, not only because of its great structural diversity but also because of the great number of possible applications, catalysis, new drugs, magnetism, among others.
At present, one of the important research topics is to relate the structure of the compounds with the properties, in order to be able to design molecules that have a certain application.
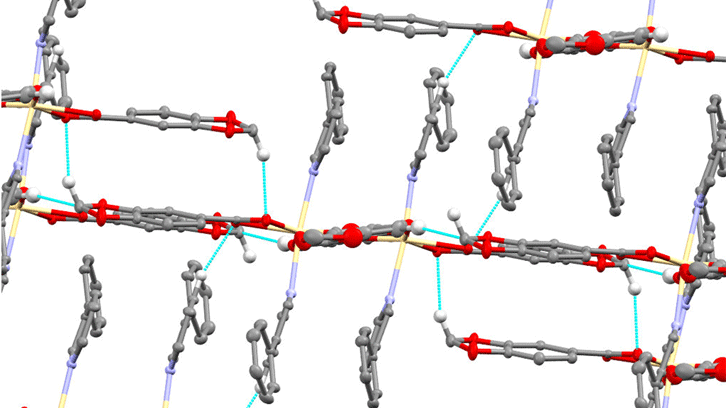
In this subject not only the molecular structure is important but also the spatial structure, which is defined by the interactions between molecules (1D, 2D, 3D). In these interactions also the solvent molecules (water, methanol, ethanol ...) that these compounds can contain are very important.
In this work, it is intended to design compounds based on carboxylated ligands (XCOO-) and pyridine-amines of large size, in order to test their reactivity with zinc acetate and cadmium acetate.
The results obtained are very promising since zinc and cadmium compounds have been obtained with different formula, which indicates that the radius of the metal is a determining factor for the compounds to have certain properties.
In this work, four compounds, two zinc and two cadmium, have been synthesized. They have been characterized by different analytical and spectroscopic techniques, in addition have been obtained crystals that have been able to be solved by X-Ray Diffraction.
This has allowed us to know both the molecular structure and the supramolecular (3D) structure, their behavior as photoluminescent compounds was also tested, observing that cadmium compounds are more photoluminescent than zinc ones, and that luminescence also depends on the pyridyl- Amine used.
References
M. Guerrero, S. Vázquez, José A. Ayllón, Teresa Calvet, Merce Font-Bardia, Josefina Pons. Zinc(II) and cadmium(II) coordination dimers based on mixed benzodioxole-carboxylate and N-donor ligands: synthesis, characterization, crystal structures and photoluminescence properties. ChemistrySelect, 2017, 2, pp632-639. DOI: 10.1002/slct.201602017.

