Discovery of an Environmental Mycobacterium with Antitumour Capacity
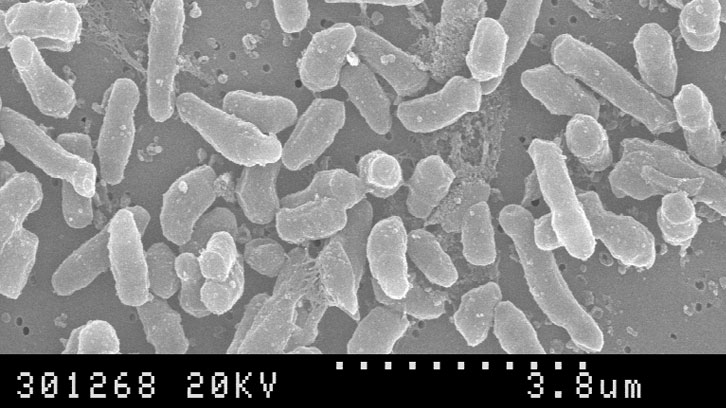
The administration of a live bacterium, Mycobacterium bovis BCG, is the current treatment for superficial bladder cancer patients after tumour resection. The intravesical treatment using this mycobacterium prevents tumour recurrence and progression. Although BCG treatment is efficacious, several adverse events are frequently reported including infections due to BCG.
The Mycobacteria Research Group at the UAB has discovered the antitumour capacity of Mycobacterium brumae. The results of the preclinical studies indicate that M. brumae inhibits tumour cell proliferation and it is able to activate the immune system towards an antitumour profile. Moreover, the fact that M. brumae is not pathogenic leads to considering this environmental mycobacterium an ideal candidate to replace BCG for bladder cancer treatment.
Mycobacteria are the only bacteria used in antitumour immunotherapy. Specifically, Mycobacterium bovis bacillus Calmette-Guerin (BCG), which is also used as vaccine for tuberculosis, is intravesically administrated for the treatment of non-muscle invasive bladder cancer patients after tumour resection, since more than 25 years ago. This treatment prevents tumour recurrence and progression to more aggressive stages. Although clearly efficacious as an immunotherapeutic tool, BCG is not a perfect treatment. Numerous adverse events, including infection due to BCG instillation, are reported. These cases should be treated with antituberculous drugs.
In the work done during the last seven years, led by Dra. Esther Julián from the Mycobacteria Research Laboratory, at the Department of Genetics and Microbiology of the UAB, including a wide group of mycobacteria species has led to the discovery of the antitumour capability of an environmental mycobacterium: Mycobacterium brumae.
The in vitro studies have shown that M. brumae is capable of inhibiting tumour cell proliferation. It is able to reduce the growth of both high and low-grade tumour cells without affecting the non tumoral cells. In addition, M. brumae is able to stimulate the immune system (i.e., activating antigen-presenting cells and inducing the production of proinflammatory cytokines), triggering a cytotoxic response against bladder cancer cells. Finally, preclinical studies using the orthotopic murine model of bladder cancer has showed M. brumae’s efficacy in the treatment of the disease. Mice intravesically treated with M. brumae, as is done in bladder cancer patients, survive longer that non-treated mice, and even in a higher proportion than mice treated with BCG.
The work done at the UAB also demonstrates the non-pathogenicity of M. brumae, which, in contrast to BCG, does not remain in treated animals, pointing out that M. brumae could be a safer immunotherapeutic tool than BCG.
M. brumae is a fast-growing environmental mycobacterium. Thus, M. brumae is safer and faster to produce than BCG. It is worth mentioning that the recent supply shortages of BCG worldwide have highlighted its costly production, due mainly to its slow growth. Shortages of BCG have persisted for more than two years, affecting seriously the treatment of bladder cancer patients. Overall, our results indicate that M. brumae represents a promising alternative to the BCG treatment for bladder cancer patients.
This work has been done in collaboration with Dr Rosa M. Rabanal of the Murine and Comparative Pathology Unit of the Department of Animal Medicine and Surgery at the UAB, and the Bacterial Infections and Antimicrobial Therapies group of the Catalan Institute of Bioengineering (IBEC), led by Dr Eduard Torrents.
Department of Genetics and Microbiology
References
Noguera-Ortega, E.; Secanella-Fandos, S.; Eraña, H.; Gasión, J.; Rabanal, R. M.; Luquin, M.; Torrents, E.; Julián, E. The non-pathogenic Mycobacterium brumae inhibits bladder cancer growth in vitro, ex vivo, and in vivo. European Urology FOCUS. 2015. doi: 10.1016/j.euf.2015.03.003.

