Fluorescent efficiency of three m-carborane-anthracene derivatives
Anthracene derivatives are molecules with excellent luminescence properties, which make them perfect scaffolds for optical applications. Furthermore, carborane clusters, C2B10H12, are fascinating chemical species characterized by a unique 3D structure with σ-delocalization, high thermal and chemical stability, hydrophobicity and low toxicity in biological systems. The ortho- (1,2-C2B10H12) and meta-carborane (1,7-C2B10H12) isomers have different electronic properties, among them, their electron-withdrawing capacity.
Our group has demonstrated that in carborane-containing fluorophores, the emission properties in solution depend critically on the cluster isomer and the fluorescence efficiency can be tailored by changing the substituent at the second C atom of the cluster (Cc). Noticeably, the m-carborane is a perfect platform to boost the photoluminescent properties of organic π-conjugated systems linked to it in both, solution and solid states. In the last decade, the development of boron cluster-based organic π-conjugated systems have attracted huge interest as active materials in (opto)electronic devices, solar cells, biological sensors and imaging. Fluorescence imaging techniques have been used to visualize bio-components and bio-processes by transforming the chemical and biological information into detectable signals.
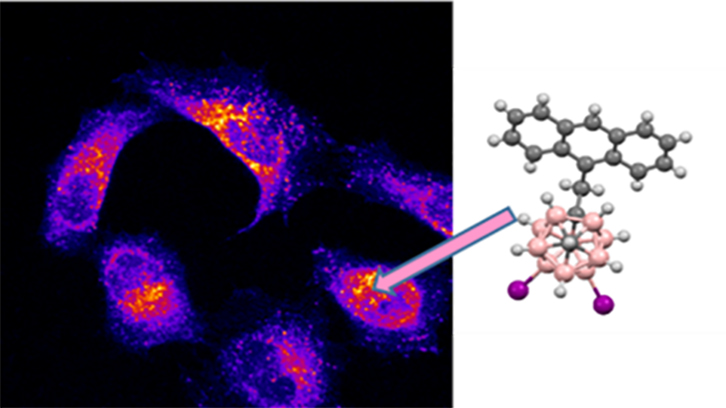
Owing to our interest in using luminescent carborane derivatives as fluorescence probes for biological systems, in this work we have developed efficient blue light emitting materials by combining the properties of the anthracene and m-carborane, and we have analysed their cytotoxicity and cellular uptake. For this purpose, we have synthesized three m-carborane-anthracene dyads, in which the carborane is non-iodinated, mono-iodinated or di-iodinated at the boron atoms, and the anthracene fragment is linked to one Cc through a CH2 spacer.
All the molecules exhibit exceptional fluorescence properties with very high quantum yields of around 100% in solution with emission maxima of around 415 nm, which corresponds to the emission of the anthracene group. These results indicate that by simply linking the m-carborane fragment to one anthracene unit produces a significant enhancement of the fluorescence in the final compounds. Notably, the three conjugates show good fluorescence efficiency in aggregate state with quantum yields in the range 19–23%. All these results indicate that our dyads are extremely good emitters in solution while maintaining the emission properties in the aggregate state.
None of the compounds shows cytotoxicity at different concentrations for HeLa cells. Confocal microscopy studies confirm that, although compounds exhibit a high fluorescence intensity emission, the one with two iodine groups is the best-internalized by cells. This suggests that the presence of iodine leads to a more efficient transport across the plasma membrane and a better cellular uptake of the compounds. It is also demonstrated that cell internalization is done by endocytosis, an energy-dependent (ATP consuming) process in which extracellular molecules are internalized.
In conclusion, this compound is an excellent candidate as a fluorescent dye for bioimaging studies in fixed cells. Furthermore, due to the high boron content and exceptional cellular uptake of our compounds, they could be used as potential anticancer agents for Boron Neutron Cancer Therapy (BNCT).
1. Institut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus UAB.
2. Departament de Biologia Cel·lular, Fisiologia i Immunologia (UAB)
References
Mahdi Chaari, Zsolt Kelemen, Duane Choquesillo-Lazarte, Nerea Gaztelumendi, Francesc Teixidor, Clara Viñas, Carme Nogués, Rosario Núñez. Efficient blue light emitting materials based on m-carborane-anthracene dyads. Structure, photophysics and bioimaging studies. Biomaterials Science, 2019, 7, 5324.

