Four coordination compounds resulting from a reaction between a ligand and three metals of the group 12
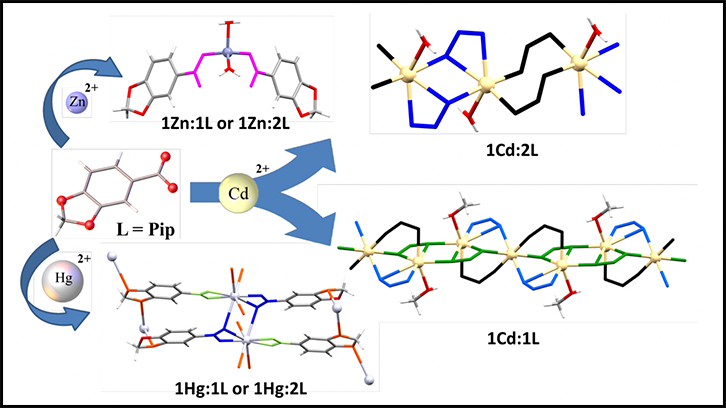
Four coordination compounds -one monomer and three polymers (CPs)- with different structures, numbers of coordination and dimensionalities are obtained in the following article. They are the result of the reaction between the ligand HPip with M, the Zn(II), Cd(II) and Hg(II) metal ions, each with their properties and abilities. They all have also been characterized and their crystalline and supramolecular structures have been obtained, among other parameters and properties analysed.
The design of one-, two-, or three-dimensional frameworks by a ligand to metal approach represents one of the most promising modern research areas. One of these structures are the coordination polymers (CPs).
CPs have attracted great interest in the past two decades for their versatile structures and their potential applications in gas storage, energy storage, host-guest chemistry, catalysis or luminescence. There are many factors that dictate the final structural disposition as the ionic counterions, the noncovalent forces, the metal/linker ratio, the temperature, or the solvent polarity, among others.
Zn(II), Cd(II) and Hg(II) as metal d10 ions favor the formation of different geometries. Cd(II) and Hg(II) centers have a larger ionic radius that allows flexibility in coordination numbers and the Zn(II) and Cd(II) CPs gained much interest for their ability to form bonds and their extraordinary physical properties. In spite of this, there have been reported few reported compounds of Hg(II) CPs containing aromatic carboxylates. Affecting all compounds, their structural feature produces low solubility complexes. For this reason, recently efforts are made with the finality to modify the characteristics of the ligands and to increase the solubility of the obtained compounds.
Polymer structure of the compound [Hg(PIp)2]n.
In this paper, on the one hand, we assayed the reaction of the ligand (HPip), which present a great variety of coordination numbers due to the presence of different functional groups, with M(MeCO2)2 (M = Zn(II), Cd(II), Hg(II)) in different experimental conditions. On the other hand, we have obtained four compounds with different geometries and topologies: with Zn(II) has been obtained a monomer [Zn(Pip)2(H2O)2] and with Cd(II) and Hg(II) have been obtained three coordination polymers [Cd(µ-Pip)2(H2O)]n, [Cd3(µ-Pip)6(MeOH)2]n i [Hg(µ-Pip)2]n. All of them present a great variety of coordination numbers, 4 (Zn(II)), 6, 7 (Cd(II)) and 8 (Hg(II)).
All compounds have been characterized by analytical and spectroscopic techniques, spectrometry of mases, thermogravimetric analysis, and for all compounds the X-ray crystal structure have been resolved. In addition, we analyzed and studied the supramolecular structures obtained 1D, 2D and 3D networks. Finally, the UV-Vis spectra and photoluminescence properties have been examined, and the quantum yields have been calculated. In conclusion, this study demonstrates that it is possible to obtain interesting structures with different dimensionalities.
Department of Chemistry.
Area of Inorganic Chemistry.
Universitat Autònoma de Barcelona (UAB).
References
Ejarque, D., Sánchez-Férez, F., Ayllón, J., Calvet, T., Font-Bardía, M., Pons, J. Diverse Structures and Dimensionalities in Zn(II), Cd(II), and Hg(II) Metal Complexes with Piperonilic Acid. Crystal Growth Design, 2020, 20, 383-400, https://doi.org/10.1021/acs.cgd.9b01317
Altres referències
1) M. Eddaoudi, J. Kim, N. Rosi, D.Vodak, J. Wachter, M.O’Keeffe, O. M. Yaghi, Science 2002, 295, 469-472
2) D. Zhang, Z.- Z. Xue, J. Pan, J.-H. Li, G.-M. Wang, Cryst. Growth Des. 2018, 18, 1882-1890
3) P. V. Dau, L. R. Polanco, S. M. Cohen, Dalton Trans. 2013, 42, 4013-4018
4) J. Soldevila-Sanmartín, J. A. Ayllón, T. Calvet, M. Font-Bardía, C. Domingo, J. Pons, Inorg. Chem. Commun. Inorg. Chem. Commun. 2016, 71, 90-93
5) F. Sánchez-Férez, L. Bayés, M. Font-Bardía, J. Pons, Inorg. Chim. Acta 2019, 494, 112-122

