In situ preparation of hypervalent iodine reagents for the chlorination of arenes
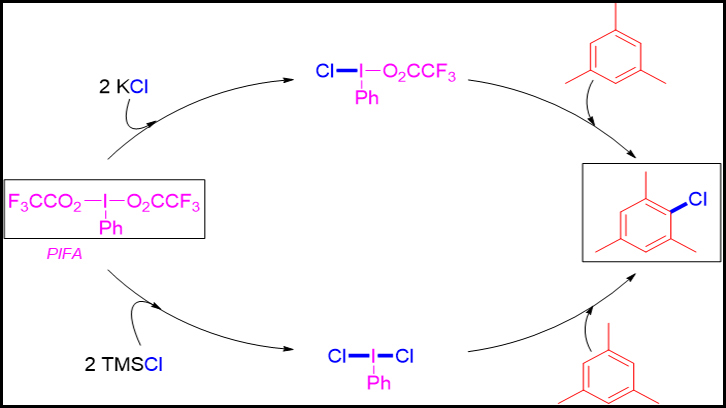
The Catalysis and New Synthetic Methodologies with Application to Nanotechnology and Materials Science research's group from UAB has designed two synthetic methodologies that combine hypervalent iodine reagent (PIFA) and a simple chlorine source to synthesize chloroarenes. We highlight the use of potassium chloride (KCl) or TMSCl as economical and non-toxic reagents. Depending on the chlorine source used, two different reactives intermediates are generated, such as: PhI(Cl)O2CCF3 ; and dichlorate: PhI(Cl)2 ( both seen in this figure).
Chloroarenes are organic compounds based on an aromatic ring with at least one chlorine atom attached to one of the carbon atoms. Chloroarenes are abundant in the pharmaceutical field, Voltaren® is a relevant example. Moreover, these compounds are precursors of more complex molecules through for example the well-known cross-coupling reactions.
Classically, these chlorinated compounds were prepared bubbling chlorine gas through the arene in the presence of an inorganic catalyst, such as iron (III) chloride. On the other hand, its preparation is also possible by the diazotization of aromatic amines with subsequent treatment with copper (I) chloride. In the literature, several methods can be found for the introduction of chlorine into aromatic systems using other metals, such as nickel, rhodium, ruthenium or palladium.
In this work, our intention was to develop a new synthetic methodology for the synthesis of chloroarenes without using toxic heavy metals, expensive organometallic catalysts or chlorine gas as reagents. For this reason, we evaluated the use of the hypervalent reagent PIFA and a simple chlorine source, such as potassium chloride, as reagents. PIFA is an iodobenzene based derivative, which iodine atom is attached through a covalent bond to one phenyl and two trifluoroacetate groups (PhI(O2CCF3)2 (figure 1). Usually iodine possesses valence I, but in PIFA’s case the iodine possesses valence III because its coordination. Simple mixing these two reagents (1 equivalent of PIFA and 2 equivalents of KCl) in dichloromethane, we demonstrated the in situ formation of a monochlorinated hypervalent iodine reagent intermediate (PhI(Cl)O2CCF3) able to transfer the chlorine atom when an arene is added. Using this methodology, we prepared up to 7 different chloroarenes with chemical yields between 35 to 100%. The best results were achieved for moderate activated electron-rich arenes. For electron rich arenes (very activated) by-products were obtained, thus decreasing the yield of the chlorinated product.
Due to the observed limitations of this method, we changed the chlorine source to trimethylsilyl chloride (TMSCl), a chlorinated organic molecule. This time, the mixture of one equivalent of PIFA and 2 equivalents of TMSCl in dichloromethane provided the in situ formation of a new hypervalent iodine reactive intermediate with two chlorine atoms attached to the iodine centre (PhI(Cl2) (figure 1). After the addition of the arene, the corresponding chloroarene was obtained successfully. Using these new conditions, the reactions resulted faster and a broad group of arenes were tolerated, such that, up to 20 different chloroarenes were prepared with chemical yields between 77 to 100%. So, we demonstrated that the in situ generated reactive intermediate PhI(Cl2) is more effective than PhI(Cl)O2CCF3.
As a conclusion, we have developed two new practical and mild synthetic methodologies based on the use of the hypervalent iodine reagent PIFA and simple chlorine sources such as KCl and TMSCl. We showed that depending the chlorinating reagent, different reaction intermediates were generated, and in consequence different reactivity was obtained. For the chlorination of moderately electron-rich arenes we recommend the use of KCl, however for an electron rich arenes TMSCl provides better results.
Chemistry Department.
Organic Chemistry Section.
Universitat Autònoma de Barcelona (UAB).
References
Granados, A., Jia, Z., Del Olmo, M., Vallribera, A. Cover Feature: In situ Generation of Hypervalent Iodine Reagents for the Electrophilic Chlorination of Arenes. European Journal of Organic Chemistry. 2019 maig; 2019 (17): 2768-2768 https://doi.org/10.1002/ejoc.201900237

