Identification of a Molecule which Would Facilitate the Action of the Antifungal Drugs
Fungal organisms are responsible for life-threatening systemic infections, fact that is especially relevant in immunocompromised patients. Current antifungals often target features that are present in the infecting organisms but are absent in humans. However, it may be also possible to target proteins present in the human host, if sufficient specificity for the fungal target can be achieved.
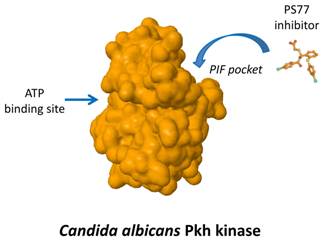
We are interested in the specific inhibition of a particular family of fungal protein kinases that is similar to the human phosphoinositide-dependent protein kinase 1 (PDK1). Human PDK1 activates a group of other kinases that play essential roles in regulating processes related to cell growth, proliferation, survival and metabolism. The interaction between PDK1 and some of its substrates occurs by a particular regulatory sequence called PIF-pocket.
We have used the budding yeast S. cerevisiae as a model of other pathogenic yeast, such as C. albicans, because both yeast species possess a family of proteins kinases similar to human PDK1, termed Pkh, that are essential for the yeast survival. Fungal Pkh and human PDK1 are not only similar proteins but the last can exert the functions of the former. It has been shown that Pkh proteins also have multiple roles in yeast cells, and we have demonstrated that reduced levels of yeast Pkh proteins causes hypersensitivity to compounds that alters the yeast cell wall and that absence of Pkh triggers a programmed cell death process.
Most drugs directed to protein kinases target a sequence needed for the binding to ATP, a compound essential for the kinase function. Due to the similarity of this site among most of the protein kinases of multiple species, such compounds are often too unspecific. Thus, compounds targeting the ATP-binding site of fungal Pkh are expected not only to inhibit human PDK1 but also to inhibit multiple additional human kinases, producing unwanted side effects.
We have identified relevant differences between the PIF pocket of human PDK1 and the one of yeast Pkh proteins. This fact allowed us to postulate that targeting fungal PIF pocket will be a good strategy to achieve high specificity. In this work we have described a novel small compound, PS77, which specifically binds to the C. albicans PIF-pocket and preferentially inhibits Pkh from C. albicans over human PDK1.
Together, our results sheds light on 1) the potential of Pkh of C. albicans as antifungal drug target, 2) the possible use of Pkh inhibitors in combination with drugs targeting the integrity of the cell wall to synergize the effects in fungal infectious organisms, and 3) the PIF-pocket regulatory site of C. albicans Pkh provides the desired selectivity to distinguish its Pkh from human PDK1 for selective antifungal drugs.
At any rate, PS77 represents a proof-of-principle that can be considered as a starting point for drug development. Similar strategies targeting allosteric sites may be envisaged for other kinases and other organisms producing fungal infections.
References
Pastor-Flores, D.; Schulze, J.O.; Bahí, A.; Giacometti, R.; Ferrer-Dalmau, J.; Passeron, S.; Engel, M.; Süß, E.; Casamayor, A.; Biondi, R.M. PIF-Pocket as a Target for C. albicans Pkh Selective Inhibitors. ACS Chemical Biology 8 (10): 2283–2292. 2013.

