New cardiac reparative therapy developed for myocardial infarction scars
The cardiovascular research group at the Germans Trias i Pujol Research Institute has developed a new strategy to regenerate cardiac scars caused by myocardial infarction. They are now studying whether this therapy, which reduces infarct size and improves cardiac function, also protects against malignant ventricular arrhythmias.
Despite the recent improvements in cardiac revascularization strategies, myocardial infarction continues to be the main cause of morbidity and mortality worldwide. In this sense, several types of cardiac regeneration therapies have emerged with the aim of repairing myocardial infarction scars. Our group of cardiovascular research (ICREC, Germans Trias i Pujol Research Institute) has developed a cardiac reparative therapy called AGTP (Adipose Graft Transposition Procedure) which consists of applying an adipose pedicle of autologous pericardium over the myocardial scar, preserving its vascularization. This therapy has already been shown to reduce the size of the infarct and improve cardiac function, but the impact of AGTP on arrhythmogenesis has not been addressed. Given that one of the main causes of mortality derived from myocardial infarction is the arrhythmic cause due to malignant ventricular arrhythmias, we carried out the study: “Electrophysiological effects of adipose graft transposition procedure (AGTP) on the post-myocardial infarction scar: A multimodal characterization of arrhythmogenic substrate”, with the objective of evaluating the effect of AGTP therapy in terms of arrhythmic safety in an animal model (porcine).
It was a prospective and randomized study. We included 20 animals, all of whom underwent a myocardial infarction by coil embolization of the first marginal branch of the circumflex coronary artery. At 15 days post-infarction, we performed an initial baseline assessment of the arrhythmic substrate in all subjects, which consisted of:
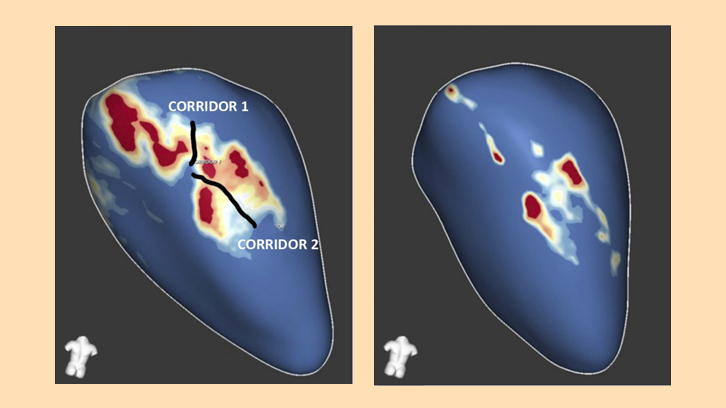
- A cardiac magnetic resonance analyzed by a post-processing dedicated software, which allows the quantification of total scar mass and its subtypes (dense scar and heterogeneous scar or border zone) and identifying the potential slow conduction channels (crucial elements for the occurrence of ventricular tachycardia circuits).
- A high-density electroanatomical mapping of the left ventricle, for the evaluation of the voltage, activation and conduction velocity of the ventricular myocardial tissue.
- Programmed ventricular pacing for the induction of ventricular arrhythmias.
Immediately after the first evaluation, the subjects were randomized 1:1 into two groups: the group treated with AGTP and the control group (whose subjects underwent thoracotomy and pericardiectomy without transposition of the adipose pedicle). A 15-days Holter-ECG was placed to detect arrhythmias in subjects of both groups. One month after surgery, the animals underwent a second evaluation of the arrhythmic substrate with the same tests performed in the baseline assessment. At the end of the study, an analysis of gene and protein expression of myocardial cell receptors involved in electrical conduction and induction of arrhythmias was performed.
The AGTP group presented a significant reduction in the areas of heterogeneous scar, slow conduction, deceleration of electrical conduction and signal fractionation compared to the control group. All these parameters had shown in previous studies to be involved in the genesis and maintenance of ventricular arrhythmias. There were no differences in the number of induced or spontaneous ventricular arrhythmias in the electrophysiological study or Holter-ECG monitoring. SERCA2, Cx43, and RyR2 gene expression was reduced in the dense scar tissue of AGTP-treated animals, indicating greater tissue homogeneity in that group.
In conclusion, treatment with AGTP of non-reperfused chronic MI is a safe reparative therapy in terms of arrhythmic risk and provides additional protective effect against adverse electrophysiological remodeling by homogenizing the scar, reducing border zone components, and improving areas of slow conduction and fractionation.
ICREC Research Program, Germans Trias i Pujol Research Institute (IGTP)
Antoni Bayés
Heart Institute (iCOR), Germans Trias i Pujol University Hospital
CIBER Cardiovascular, Instituto de Salud Carlos III
Department of Medicine, Can Ruti Campus, Universitat Autònoma de Barcelona
References
Adeliño R, Martínez-Falguera D, Curiel C, Teis A, Marsal R, Rodríguez-Leor O, Prat-Vidal C, Fadeuilhe E, Aranyó J, Revuelta-López E, Sarrias A, Bazan V, Andrés-Cordón JF, Roura S, Villuendas R, Lupón J, Bayes-Genis A, Gálvez-Montón C and Bisbal F (2022) Electrophysiological effects of adipose graft transposition procedure (AGTP) on the post-myocardial infarction scar: A multimodal characterization of arrhythmogenic substrate. Front. Cardiovasc. Med. 9:983001. doi: 10.3389/fcvm.2022.983001

