Revealing different regenerative responses in peripheral neurons after nerve injury
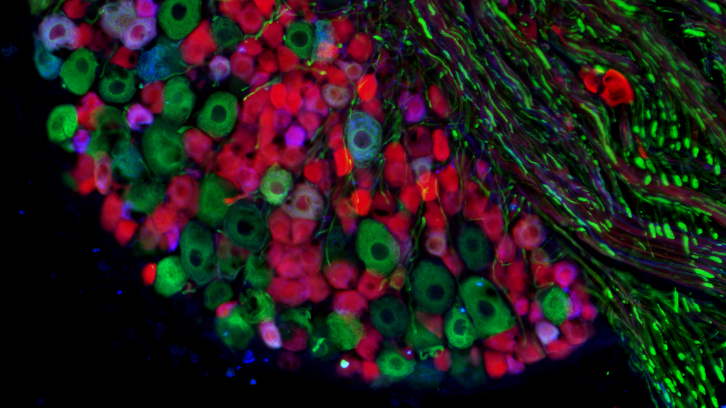
Researchers from the Institut de Neurociències of the UAB have identified key similitudes and differences in the regenerative capacity of four subtypes of peripheral neurons after nerve injuries using transgenic mice. This knowledge may help to develop specific strategies to improve functional recovery after nerve injury in patients.
Severe injuries of peripheral nerves can have devasting functional consequences for patients, being their recovery limited due in part to the inaccuracy of reinnervation. Nerves are formed of the axons of motoneurons that innervate skeletal muscles and different types of sensory axons that innervate both the skin and the muscles. Injuries on these nerves can disrupt axons, and the distal segment would degenerate, with the consequent loss of function of the denervated territory.
Luckily, these axons can regenerate and eventually reinnervate target organs, although this reinnervation must be accurate for full functional recovery. Motor axons and sensory axons carrying sensation from muscles (proprioception) have to grow through the muscular branch, whereas tactile, pain and thermal sensory axons have to mainly grow through the cutaneous branch.
Consequently, when designing strategies to enhance nerve regeneration, the specificity of each type of neuron must be taken into account with the final aim of promoting accurate reinnervation and, thus, a better functional recovery. A better knowledge of the intrinsic capabilities of different subtypes of peripheral neurons can help us find therapies to improve functional outcomes after these injuries.
Our paper aims to investigate the differences among neuron subtypes and to highlight the importance of specifically evaluating and modulating the growth of these populations as a first step to design strategies to improve reinnervation accuracy. Therefore, we have addressed two main questions: Is the intrinsic growth capacity of 4 paradigmatic subtypes of peripheral neurons different? Could these intrinsic differences help us design strategies to specifically promote the regeneration of these subtypes of neurons?
By taking advantage of transgenic mice, we evaluate the regenerative capability and the genetic program activated after injury by motoneurons, proprioceptive (position) neurons, nociceptive (pain) neurons and a subpopulation of mechanoreceptors, that include both touch and nociceptive neurons. In vivo, we measured the amount of regeneration and observed that nociceptive axons were the fastest, followed by motoneurons and cutaneous mechanoreceptors, being proprioceptors the slowest ones.
When analyzing the different genetic programs activated by these neurons after nerve injury, we observed that only 20% of the activated genes were shared between all populations, suggesting a common regenerative pattern, but also activation of specific pathways in each population. Focusing on key pathways in vitro, we could promote the selective growth of different sensory populations, either by adding trophic factors or manipulating gene expression.
Therefore, we can answer the two questions by saying that yes, the intrinsic growth capacity of these subtypes of peripheral neurons is different, and yes, these intrinsic differences can help us design strategies to specifically promote the regeneration of these subpopulations.
Sara Bolivar Martin; Esther Udina Bonet
Neuroplasticitat I Regeneration Group
Department of Cell Biology, Physiology and Immunology
Institut de Neurociències, Facultad de Medicina
Universitat Autònoma de Barcelona
References
Bolívar S, Sanz E, Ovelleiro D, Zochodne DW, Udina E. Neuron-specific RNA-sequencing reveals different responses in peripheral neurons after nerve injury. Elife. 2024 May 14;12:RP91316. doi: 10.7554/eLife.91316. PMID: 38742628; PMCID: PMC11093584.

