Visible-light mediated method for the synthesis of medicinally and agriculturally relevant phosphonates
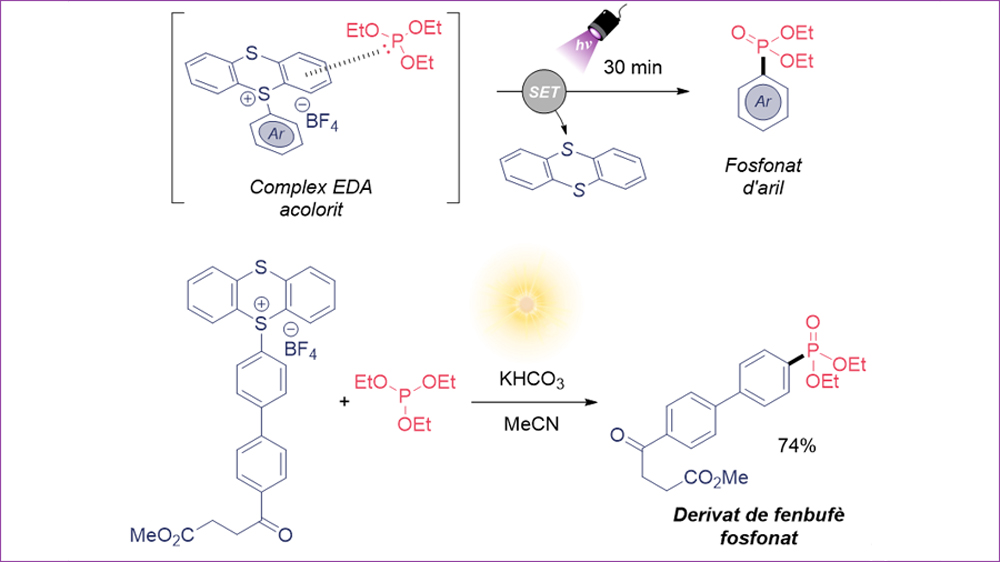
Nowadays, redox processes that occur via visible light (known as photoredox catalysis) represent a great tool for the development of new synthetic methods. These processes usually produce radical species (species with one unpaired electron in the shells around the atomic nucleus) through single-electron transfer (SET) steps. Historically, photoredox catalysis uses metal complexes of ruthenium or iridium as photocatalysts, which have the capacity to absorb visible light energy to subsequently produce SET processes, thus opening a way to new reactivity. Despite the efficiency of these photocatalysts, it is desirable to avoid them given their high cost. An interesting strategy that avoids the use of catalysts is the combination of an electron acceptor molecule with an electron donor molecule. In these cases, both molecules form a new aggregate known as electron donor-acceptor complex (EDA), which typically presents coloration and presents very specific redox and spectroscopic properties. Notably, these complexes can absorb visible light and engage in SET processes that allow the generation of radical species.
Interesting compounds that have proven to be excellent electron acceptor species in EDA strategies are the so-called aryl thianthrenium salts. These salts are formed by a benzene ring attached to a sulfur atom of the thianthrene unit (cationic part) and a counterion, typically tetrafluoroborate (BF4-).
Aryl phosphonates are very interesting in medicinal and agricultural chemistry, thus our research group proposed using thianthrene salts (acceptors) together with triethyl phosphite (P(OEt)3, donor) for the formation of a new EDA (see Figure) complex, which ultimately allows the formation of aryl phosphonates using visible light. To our delight, when combining both reagents we observed that the solution presented color.
The optimization process allowed obtaining the desired aryl phosphonate in excellent yield using potassium bicarbonate in acetonitrile and under irradiation of a 390 nm LED for only 30 minutes. This method has been applied to a large number of substrates, where we highlight electron-rich and electron-poor aromatic rings, as well as molecules derived from other biologically active ones such as the anti-inflammatory Fenbufen. Notably, this method also works perfectly using sunlight as light source (74% yield in Figure).
As summary, our group have developed a practical and efficient synthetic method for the preparation of aryl phosphonates. We have demonstrated the formation of a new EDA complex based on aryl thianthrene salts and phosphites. Finally, this method could serve as an efficient tool for the phosphonation of biomolecules.
Departament of Chemistry
Universitat Autònoma de Barcelona
References
Gallego-Gamo Albert, Reyes-Mesa David, Guinart-Guillem Axel, Pleixats Roser, Gimbert-Suriñach Carolina, Vallribera Adelina, Granados Albert. Site-selective and metal-free C–H phosphonation of arenes via photoactivation of thianthrenium salts. 2023. RSC Advances. http://dx.doi.org/10.1039/D3RA04512A


