Water-Soluble Gold Nanoparticles as Catalyst for the Selective Reduction of Organic Compounds in Water
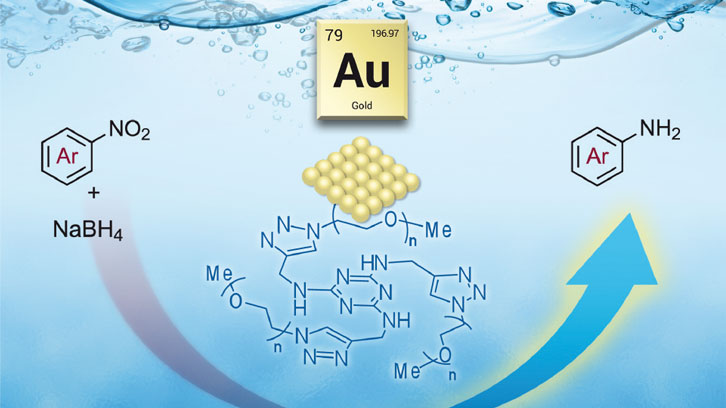
The scientific community is particularly interested in the development of chemical processes with low cost and minimal environmental impact. The use of water as a solvent and the design of recyclable catalysts to increase the rate of chemical reactions with minimal waste are some of the promising directions in this field. Among water-soluble catalysts, a great deal of attention has been focused on gold nanoparticles because of its size-related properties. Moreover, gold nanoparticles may also have other potential applications in the fields of biomedicine and sensors.
A wide range of stabilizers for the preparation of metal nanoparticles are known, in order to prevent their aggregation. The nature of the protecting agent determines the solubility of the resulting nanomaterial. In this context, our work has focused on the use of a nitrogen-rich polyethylene glycol (PEG)-tagged derivative as stabilizing agent for gold nanoparticles. The PEG chains are commercially available in various molecular weights, are soluble in water and are insoluble in diethyl ether.
In this work, a new nitrogen-rich substrate bearing longer polyether chains was synthesized and used as stabilizer for the preparation of water-soluble gold nanoparticles. The newly prepared and characterized nanomaterial displayed good activity as catalyst for the mild and selective reduction of a type of organic compound, called nitroarenes, in water at room temperature. It should be noted that the recyclability of the nanocatalyst was established, taking advantage of its solubility properties, providing good isolated yields in successive runs.
These nanoparticles were also successfully tested as refractive index sensor. Gold nanoparticles absorb light of a specific wavelength, which directly depends on the index of refraction of the environment or solvent which wraps the nanoparticles. When the sensor (gold nanoparticles) was placed in different solvents, it was observed a linear correlation between the response (wavelength of the maximum of absorption) and the refractive index of the solvent.
Image 1: Solutions of different nanomaterials (gold nanoparticles) in water. The color of the solution depends on the particle size.
References
Guo, Wusheng; Pleixats, Roser; Shafir, Alexandr. Water-soluble gold nanoparticles: from catalytic selective nitroarene reduction in water to refractive index sensing. Chemistry, an Asian Journal. 2015, vol. 10, num. 11, p. 2437-2443. doi: 10.1002/asia.201500995.

