Cellular Response to Protein Misfolding
Protein misfolding and aggregation is associated with numerous degenerative human disorders such as type-II-diabetes or Alzheimer’s disease. Despite this, aggregation prone proteins seem to be conserved in all kingdoms of live. In fact, there is an increasing number of examples in which cells exploit protein aggregates for functional purposes, from scaffold the melanin in the skin to memory storage. Consequently, during evolution cells have developed different strategies to tolerate and control the protein aggregation process, such as control the protein expression or chaperones to facilitate a correct protein folding.
One of the main research lines of our group is study the misfolding and aggregation of disease-related proteins. We study this phenomenon from a biophysics point of view to know the properties involved in this event but also using in vivo models to understand its relationship with diseases and cellular functions.
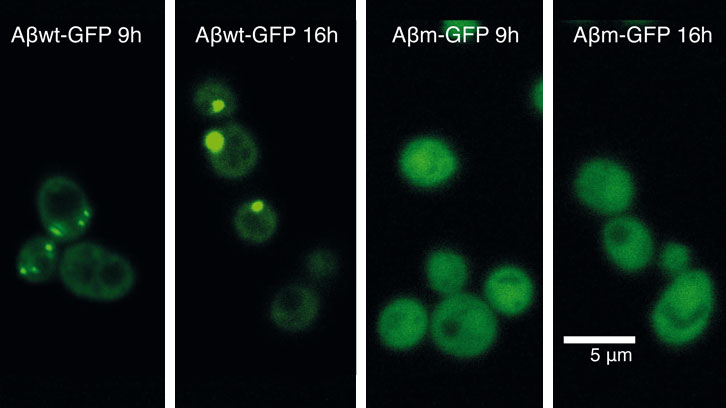
As a first attempt to unravel how cells response against protein misfolding, we recently expressed 20 GFP-fused peptides covering a continuous range of aggregation propensities. We derived these peptides from the amyloid-β-peptide (Aβ42) (associated to Alzheimer disease) and expressed them in yeast. Interestingly, despite that most of these peptides are highly insoluble, just some of them are recruited into foci. With this approach, we identified an aggregation propensity threshold above which the cell actively accumulates a protein into foci.
Now, we have used two proteins from this collection, which are located on either side of the aggregation threshold, to decipher why protein foci are or are not formed in cells. Specifically, we have characterized how these two proteins impact on cell fitness and cellular homeostasis.
|
|
Figure 2: One unfolded protein response: two strategies to control the toxic effects. Model showing the different benefits and costs of accumulating a misfolded protein into foci. Specifically, this diagram shows two proteins, mostly insoluble, but located either side of an aggregation threshold above which the cell actively recruits a protein into foci [11] (a). This process could offer benefits but it is also energetically expensive (cost). Under the threshold, the protein remains diffuse through the cytoplasm (b). This could favour the formation of harmful interactions that could initiate a cascade of misfolding and oxidative stress. ROS, reactive oxygen species. |
Institut de Biotecnologia i Biomedicina (IBB-UAB)
References
Sanchez de Groot, N.; Gomes, R. A.; Villar-Pique, A.; Babu, M. M.; Coelho, A. V.; Ventura, S. Proteome response at the edge of protein aggregation. Open Biology. 2015, vol. 5, num. 2, p. 140221. doi: 10.1098/rsob.140221.
Villar-Pique, A.; Ventura, S. Protein aggregation propensity is a crucial determinant of intracellular inclusion formation and quality control degradation. Biochimica et Biophysica Acta. 2013, vol. 1833, num. 12, p. 2714-2724. doi: 10. 1016/j.bbamcr.2013.06.023.
Sanchez de Groot, N.; Torrent, M.; Villar-Pique, A.; Lang, B.; Ventura, S.; Gsponer, J.; Babu, M. M. Evolutionary selection for protein aggregation. Biochemical Society transactions. 2012, vol. 40, num. 5, p. 1032-1037. doi: 10. 1042/BST20120160.
De Groot, N. S.; Aviles, F. X.; Vendrell, J.; Ventura, S. Mutagenesis of the central hydrophobic cluster in Abeta42 Alzheimer’s peptide. Side-chain properties correlate with aggregation propensities. FEBS Journal. 2006, vol. 273, num. 3, p. 658-668. doi: 10.1111/j.1742-4658.2005.05102.x.

