Non-invasive detection of immune system participation in therapy response in an immunocompetent glioblastoma preclinical model
Researchers from the Biomedical Applications Group from the RMN (GABRMN) at the Universitat Autònoma de Barcelona have used non-invasive medical imaging approaches based in magnetic resonance, followed by machine learning analysis, in order to obtain therapy response biomarkers for glioblastoma preclinical murine models. Moreover, in this study, more than a half of the studied animals were cured by the therapeutic protocol used, with development of anti-tumour immune memory. This strategy could have translational potential for patients.
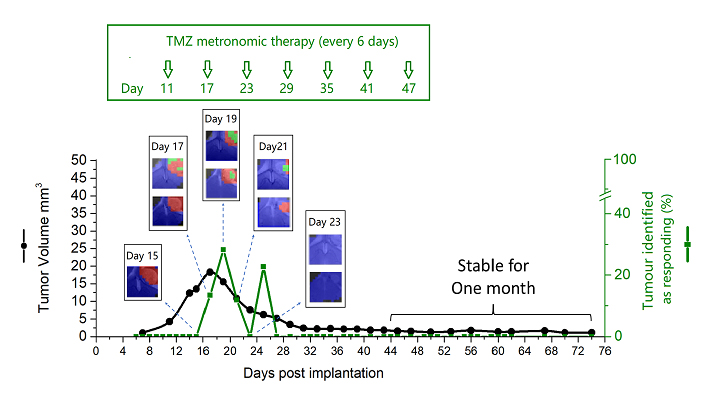
Figure: Evolution of tumour identified as responding (%) and tumour volume after Immune-Enhancing Metronomic Schedule (IMS-TMZ) treatment. Cured mice established anti-tumour immune memory and no tumour growth was seen after GL261 cell re-inoculation. Images obtained with non-invasive magnetic resonance-based techniques and machine learning analysis.
Malignant brain tumours, such as glioblastoma, have a huge impact in the quality of life of patients and, due to the lack of an efficient treatment, survival is not beyond one and a half years. Due to their location, it is not feasible to perform repeated biopsies in patients in order to check effectiveness of treatment. Thus, follow-up is usually done with non-invasive techniques, mainly magnetic resonance imaging (and also spectroscopy), although the spectroscopic information is complex and usually requires additional processing to be useful and meaningful for radiologists. On the other hand, the participation of host immune response in cancer therapy is being increasingly acknowledged. However, non-invasive follow-up should be improved in order to know, as early as possible, whether the administered therapy is effective and the host immune system is helping outcome. Knowing that would allow clinicians to adequately manage therapy: to maintain a certain treatment or to change it to a different strategy. In order to carry out systematic studies about response to therapy, preclinical (animal) models of brain tumours were used. In our work, mice with high grade glial tumours such as glioblastoma.
In our study, we wanted to systematically follow response to therapy in mice, using non-invasive magnetic resonance-based techniques, followed by machine learning approaches for analysis of spectroscopic data. We have previously reported that it is possible to generate an “image of response” based in the spectral pattern, even before any tumour size decrease can be detected, and that this “response imaging pattern” oscillated with a 6-day period when response pattern was assessed during several weeks.
Our present results confirm the previously described oscillation in almost all studied cases which responded to treatment. Moreover, we have described the correlation of this pattern with an increase of immune system cells infiltration, such as macrophages, in samples of murine tumours analysed post sacrifice in a separate cohort of mice. On the other hand, we have studied samples of “escaping from therapy” tumours and found that they presented an increased expression of a receptor named PD-L1 which is related with the ability of some cancer types to evade the immune system attack.
One of the most relevant results obtained was the total cure of 8 mice (out of 17 studied until endpoint*). Cured mice were re-challenged again with tumour inoculation without detecting tumour regrowth. This proved immune memory development against glioblastoma due to the treatment protocol used. In addition, our data suggest that the non-invasive follow-up method used for therapy response assessment may provide a good biomarker for detecting, in vivo, efficient immune system action against tumours.
*An endpoint follow-up is performed without animal euthanization for sample studies. Animal is followed during its whole life until any of the following situations is reached: a) either the tumor regrows (in this case, animal is euthanized for humanitary purposes), or b) animal gets cured, thus no longer requiring treatment.
Shuang Wu1 (+), Pilar Calero-Pérez1, 4(+), Lucia Villamañan1(+), Nuria Arias-Ramos1,4, Martí Pumarola2,4, Sandra Ortega-Martorell3, Margarida Julià-Sapé 4,5, Carles Arús1,4,5, Ana Paula Candiota4,1,5
[+] These authors contributed equally to this work.
1 Department of Biochemistry and Molecular Biology, Biochemistry Unit from Bioscience, Universitat Autònoma de Barcelona (UAB).
2 Unit of Murine and Comparative Pathology. Department of Animal Medicine and Animal Surgery, Veterinary Faculty, UAB, Cerdanyola del Vallès, Spain.
3 Department of Applied Mathematics, Liverpool John Moores University, Liverpool, UK.
4 Center of Research in Network in Biomedical in Bioengineering, Biomaterials and Nanomedicine (CIBER‐BBN).
5 Institute of Biotechnology and Biomedicine (IBB), UAB.
Corresponding author: Ana Paula Candiota, Center of Research in Network in Biomedical in Bioengineering, Biomaterials and Nanomedicine (CIBER‐BBN). AnaPaula.Candiota@uab.cat
References
1 Delgado-Goñi T, et al. MRSI-based molecular imaging of therapy response to temozolomide in preclinical glioblastoma using source analysis. NMR Biomed. 2016;29:732-743
2 Arias-Ramos N, et al. Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment. Metabolites. 2017;7:20

