Selective drug delivery and cancer treatment
Selective drug delivery is specially desired in oncology, in which conventional therapy is majorly based on the systemic administration of untargeted chemotherapeutic drugs, associated to severe life-threatening side toxicity.
In this context, cell-targeted drug delivery requires appropriate nanoscale vehicles (usually nanoparticles of a size ranging from 10 to 100nm) for the generation of nanoconjugates that allow higher drug concentration and desired biological effect at the site action. However, the xenobiotic nature and potential toxicity of most of the nanomaterials studied for drug delivery (polymers, ceramics, metals and carbon nanotubes among others) pose severe concerns about their biosafety. Fortunately, emerging nanobiotechnologies are strongly pushing the development of fully biocompatible nanosized protein materials based on the engineering of proteins that confer self-assembling at the nanoscale.
Self-assembling protein nanoparticles show high functional versatility and can act as 'artificial viruses', combining in the same entity different functions required for an adequate drug targeting, such as the recognition of a specific tumoral marker. In this context, T22 is a highly selective cationic ligand of the cytokine receptor CXCR4, that is overexpressed in about 20 human neoplasias and that correlates with aggressiveness and metastasis.
In previous studies, we have described that self-assembling T22-GFP-H6 fusion protein generates nanoparticles in the range size of 12 to 20nm. Upon systemic administration, these nanoparticles show optimal biodistribution in colorectal cancer animal models, and also specific T22-CXCR4 mediated internalization into tumor cells. In this context, T22-GFP-H6 nanoparticles have been recently used as potent nanocarriers of conventional antitumoral drugs, and as vehicles for specific cytotoxic protein delivery in different cancer animal models, both approaches with high therapeutic impact. Nevertheless, poor endosomal escape and consequent lysosomal degradation may be a critical bottleneck for T22-GFP-H6 to efficiently deliver cytotoxic drugs into the cytoplasm, as generically observed for protein materials.
In protein-based nanoparticles, functional recruitment by protein fusion technologies might be useful to enhance the endosomal escape of T22-GFP-H6 nanoparticles, which is now moderate, in order to improve its applicability as drug carrier. In this regard, the combination of targeted peptides and endosomal escape enhancers could offer a strategy to increase the efficacy of the nanoconjugate in the target cell.
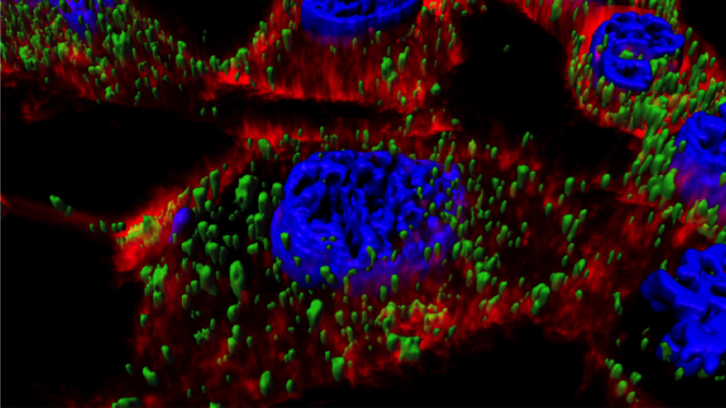
In this work, we have studied the combined activity of T22 and that of a potent pore-forming protein, the antimicrobial peptide (AMP) GWH1, simultaneously displayed on the surface of protein nanoparticles.
The data presented here demonstrate that GWH1 shows potent endosomolytic activity that increases transfection efficiently of functionalized protein nanoparticles into the cytoplasm. Despite this fact, the CXCR4 binding specificity is conserved, although reduced, in these materials.
In conclusion, the functional combination provided by the peptide pair is a promising approach to enhance the performance of smart drug nanocarriers based on functional peptides designed for cancer treatment.
Postdoctoral Researcher
Institute of Biomedicine & Biotechnology
CIBER-BBN
Universitat Autònoma de Barcelona1
Dra. Esther Vázquez
Researcher
Institute of Biomedicine & Biotechnology
Genetics and Microbiology Department
CIBER-BBN
Universitat Autònoma de Barcelona2
References
Serna, Naroa & María Sanchez, Julieta & Unzueta, Ugutz & Sanchez-Garcia, Laura & Sánchez-Chardi, Alejandro & Mangues, Ramon & Vázquez, Esther & Villaverde Villaverde, Antonio. (2018). Recruiting potent membrane penetrability in tumor cell-targeted protein-only nanoparticles. Nanotechnology, 30. DOI: 10.1088/1361-6528/aaf959.

