The chemistry of Hg (I): a structural study
Mercury (Hg) is a metal capable of forming various polycations with various geometries. In this work of the Area of Inorganic Chemistry, the synthesis and characterization of Hg2(Pip)2, a Hg (I) compound with aromatic ligands and carboxylate groups, is presented. Its characterization has been carried out by elemental analysis, spectroscopic techniques, single crystal X-ray diffraction and DFT calculations.
Hg is a metal that has the particularity of being capable of forming a variety of divalent, trivalent, or tetravalent polycations arranged either into linear, dimeric [Hg2]2+, trimeric [Hg3]2+, tetrameric [Hg4]2+, and chains [Hg]n or triangles [Hg3]4+. All of them present differences in the formation conditions, connectivity, geometry, and Hg-Hg bond lengths.
The formation of the [Hg2]2+ is stabilized by the lower solubility compared to those of their Hg(II) analogues. This preferred linear arrangement facilitates the formation of Hg···π interactions.
The coordination chemistry and structural arrangement of Hg(I) with aromatic carboxylates have not been extensively explore. In this paper, we provide a compound of Hg(I) with an aromatic carboxylate [Hg2(Pip)2] (Pip = piperonylic acid). The obtention of the compounds is due to decomposition of the [Hg(Pip)2(4,4’-bipy)]n (4,4’-bipy = 4,4’-bipyridine) to give Hg(0) that have driven the formation of the [Hg2]2+ cation. This reaction depends on the solvent, temperature, and time (in this case, dimethylformamide, 105 °C and 1h).
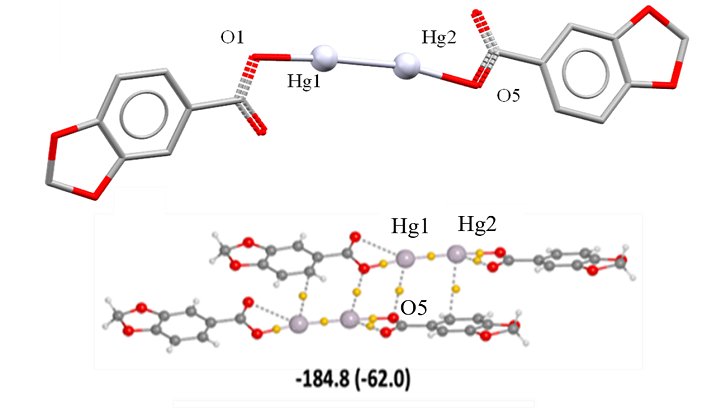
The compound obtained ([Hg2(Pip)2]), has been characterized by elemental analysis, spectroscopic techniques (IR, 1H NMR), single crystal X-ray diffraction, and DFT calculations have been performed. The compound is dimeric and the [Hg2(Pip)2] units are joined together in tetrameric [Hg4(Pip)4] assemblies by Pip ligands. The structure is expanded into a 2D supramolecular structure by C-H···O and Hg···π interactions. DFT calculations have been performed to analyze the interactions between [Hg2(Pip)2] dimers.
References
“A Hg(I) Corrugated sheet assembled by auxiliary dioxole groups and Hg···π interactions” Francisco Sánchez-Férez, Xavier Solans-Monfort, Teresa Calvet, Mercè Font-Bardia, Josefina Pons, CrystEngComm, 2022, 24, 4351-4355

